What Method of Separation Does This Describe
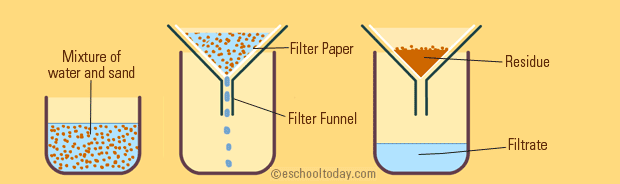
Though chromatography is a simple technique in principle it remains the most important method for the separation of mixtures into its. The liquid which has passed through the filter is called filtrate and the solid which remains on the filter paper is called the residue.

Separating Mixtures Overview Common Methods Expii
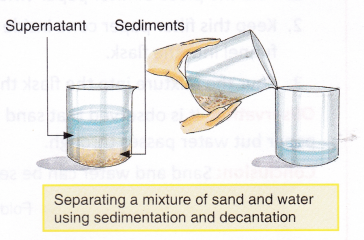
The heavier liquid which settles below is drained out first from below the funnel into a beaker and then the lighter liquid is drained out into.
. Separation by Evaporation Last Updated. 16 Aug 2021 Evaporation is the process of the separation of a solid substance that is dissolved in water. Vfe will emphasize problem solving techniques but ve must also understand how not.
What method of separation does this describe. You will have to become an expert in this method and so we will discuss quite a fev. There are also chemical methods which are used by rearranging the particles so a certain substance no longer exists chemical reaction.
Sieve plates have meshed or perforated bottoms which allow only particles of a specific size to pass through it. Gravity separation this method can separate two mixtures of different densities eg oil and water. It uses sieve plates for separation of coarse particles from finer particles.
As the name suggests evaporation is the process of conversion of water into vapour. The table shows the percentages of hydrocarbons that are found in a sample of crude oil. For example water can be separated from salty water by simple distillation.
The methods stated above are all physical methods. The application is based on the fact that solids do not vaporize easily whereas liquids do. This method works because.
1 the mixture is pored through a funnel lined with a filter paper 2 the filtrate liquid drips through to the filter flask 3 the solid remains in the funnel. Image will be uploaded soon Condensation. Learners would have also looked at some of the physical methods of separating different types of mixtures including hand sorting sieving filtration and this year we will explore some additional methods in more detail including distillation and chromatography.
Separation is an important asset to purify component of. Distillation separates a liquid from a solution. On performing evaporation the solid substance is left behind as a residue.
Separation processes or a separation method or simply a separation is methodology to attain any mass transfer phenomenon that convert a mixture of substances into two or more distinct product mixtures. Two or more substances are placed in a flask and gradually the heavier one will sink to the bottom. This method of separation of particles from a mixture based on the difference in size of particles is known as sieving.
California Institute of Technology Gas-Liquid Chromatography Many separation methods are based on chromatography that is separation of the components of a mixture by differences in the way they become distributed or partitioned between two different phases. It is oldest method of separation. The first eight sections of the chapter describe the bases for chemical separations involving oxidation-reduction complex-ion formation distillationvolatilization solvent extraction precipitation and coprecipitation electrochemistry and chromatography.
Spatial separation application when different activities are carried out in other places. Gravity Separation In gravity separation a mixture of two immiscible liquids can be separated using a separating funnel the working of which is based on the differences in the densities of the liquids. Eg places to pick up and store raw materials Factorial separation is applied when several factors contribute to the completion of the activity.
A separatory funnel is also a liquid-liquid separation mechanism that depends primarily on density andor chemical affinity. To increase particle separation efficiency a centrifugal force function to separate particles by sedimentation property. 21 Mixtures 1 hour Tasks.
In this process the mixture is passed through a filter paper. In any case it is the most dense fluid that is removed by separation from the bottom of the funnel. Nowadays chromatography is accepted as an extremely sensitive and effective separation method.
Ve -vilt use a technique called the method of separation of variables. Column chromatography is one of the useful separation and determination methods. A natural gravitational force can also concentrate particles but in an indefinite time.
An individual separation can be applied when two people need to approve before an activity is completed. It is the method of separation in which liquid solvent or organic solvent evaporates and leaves the solid residue behind. This is a very common separation technique which is used for separating an insoluble solid from a liquid.
Mixtures can be separated using various separation methods such filtrationseparating funnelsublimationsimple distillation and paper chromatography. That is immiscible compounds to not want to remain together so ionic and covalent compounds can be separated. The centrifugation method separates different components or the suspended particles from the homogenous solution through centrifugal force.
Which method would be best for separating the components of a mixture that is made from two different liquids. Hydrocarbons Percentage Paraffins 30. Absorption gas absorption can be used to separate two soluble gases bu contact with a liquid.
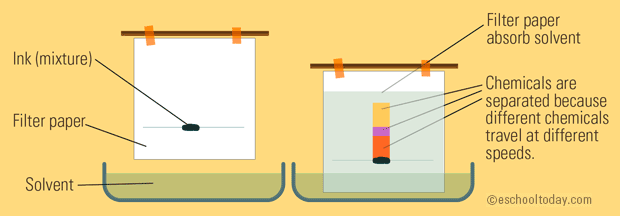
Feb 9 2015. Chromatography is the separation of a mixture by passing it in solution or suspension or as a vapor as in gas chromatography through a medium in which the components move at different rates. For example salt is obtained from seawater by evaporation.
Here are a number of common separation techniques. Thin-layer chromatography is a special type of chromatography used for separating and identifying. Column chromatography is a protein purification method realized especially based on one of the characteristic features of proteins.

Separation Of Mixtures Using Sublimation And Magnets Geeksforgeeks
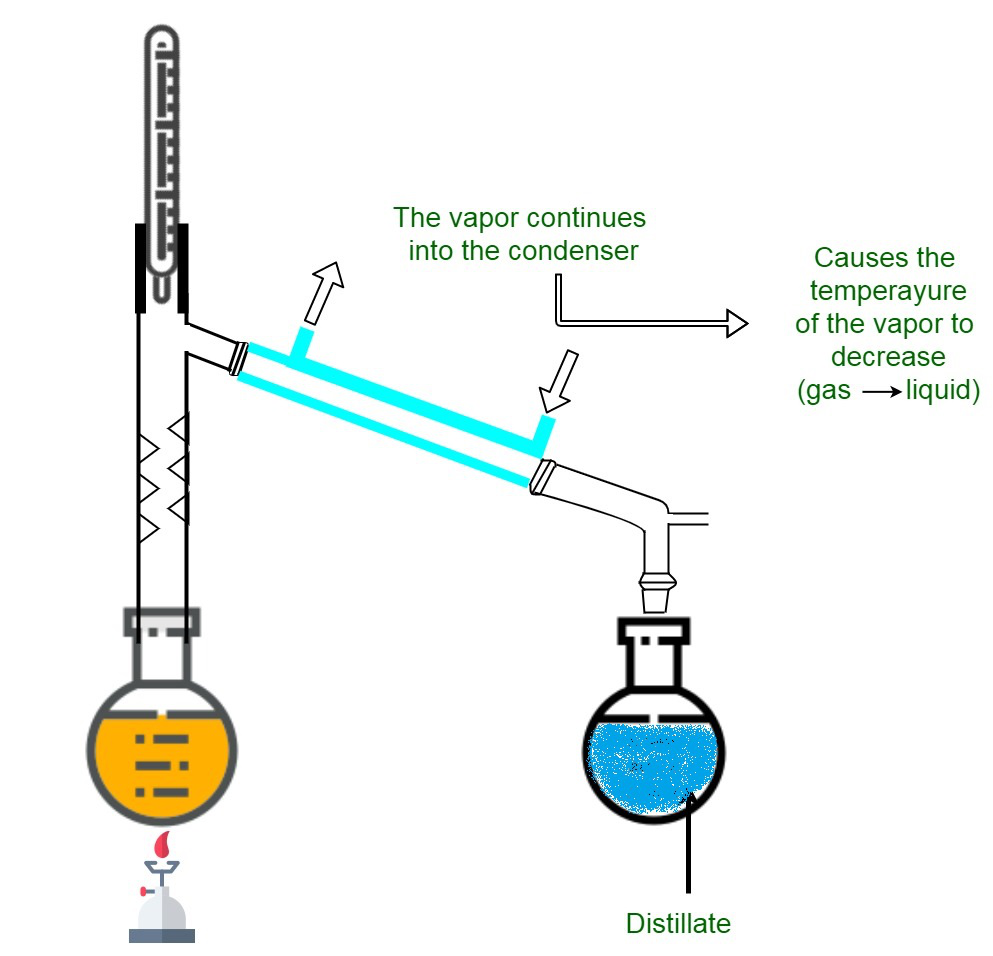
Separation By Distillation Geeksforgeeks

1 4 Laboratory Techniques For Separation Of Mixtures Chem 1114 Introduction To Chemistry
Explain The Mixture Separation Techniques Example
Explain The Mixture Separation Techniques Example
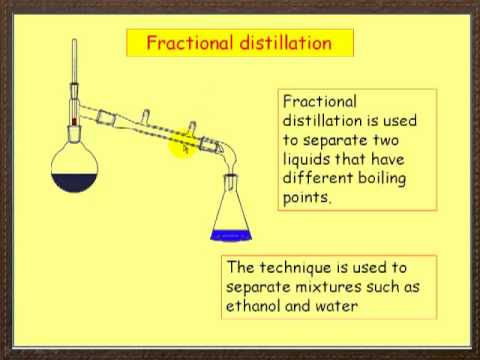
2 10 Separating Mixtures Chemistry Libretexts
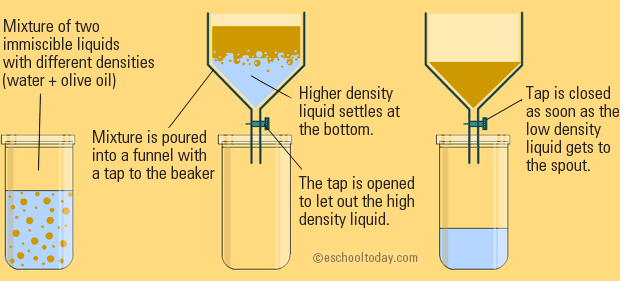
Explain The Mixture Separation Techniques Example
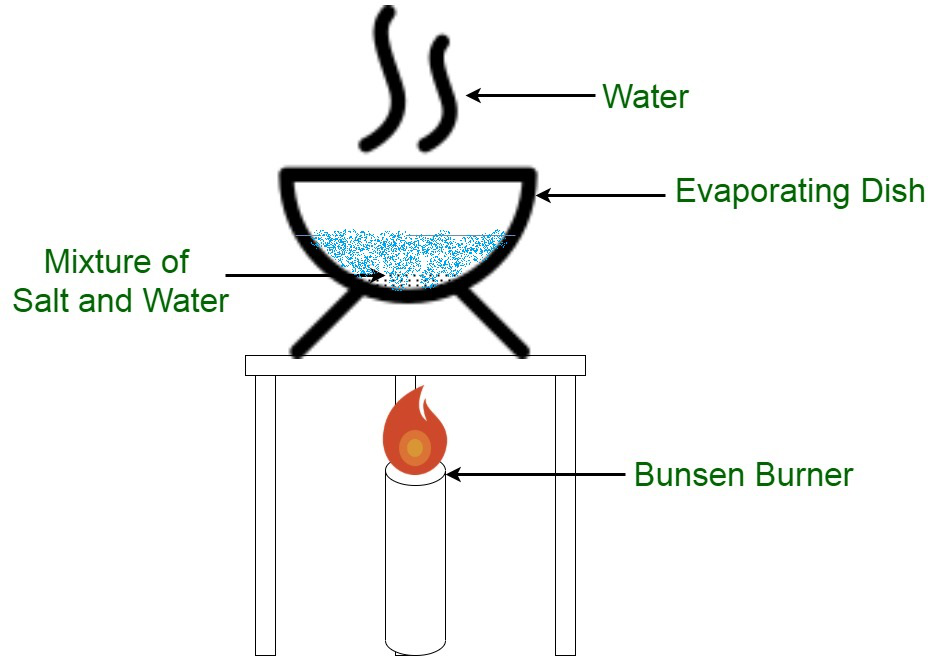
Separation By Evaporation Geeksforgeeks

Methods Of Separation Of Substances Under Wet Conditions A Plus Topper

Methods Of Separation Learn Various Separation Techniques With Examples

1 4 Laboratory Techniques For Separation Of Mixtures Chem 1114 Introduction To Chemistry

1 4 Laboratory Techniques For Separation Of Mixtures Chem 1114 Introduction To Chemistry

Methods Of Separation Learn Various Separation Techniques With Examples

Science Separation And Mixture Hindi Youtube

Separation Of Mixtures Geeksforgeeks

Separating Mixtures Overview Common Methods Expii

Methods Of Separation Learn Various Separation Techniques With Examples

Methods Of Separation Learn Various Separation Techniques With Examples

Comments
Post a Comment